New research led by UC San Francisco scientists has revealed that mutations in a gene linked with brain development may dispose people to multiple forms of psychiatric disease by changing the way brain cells communicate.
The new study — published October 18, 2016 in the journal Molecular Psychiatry — combined genetic analysis of more than 9,000 human psychiatric patients with brain imaging, electrophysiology, and pharmacological experiments in mutant mice to suggest that mutations in the gene DIXDC1 may act as a general risk factor for psychiatric disease by interfering with the way the brain regulates connections between neurons.
The new finding is the latest evidence supporting a growing precision medicine model of psychiatric disease in which disruptions of certain genes during brain development contribute to a person’s risk for multiple psychiatric disorders, with other genetic or epigenetic drivers, random developmental events, or environmental influences determining the specific disease an individual develops, said senior author Benjamin Cheyette, MD, PhD, an associate professor of psychiatry and a member of the UCSF Weill Institute for Neurosciences and the Kavli Institute for Fundamental Neuroscience at UCSF.
WNT pathway implicated as general psychiatric risk factor
Since the mid-1990s, Cheyette has been interested in the role of WNT signaling — a molecular pathway involved in early brain development and later, more refined brain wiring — in the genesis of psychiatric disorders. At the time, he says, most psychiatrists believed that psychiatric disorders were caused by imbalances in the levels of neurotransmitters in the brain.
“Antidepressant drugs worked by increasing serotonin levels. Antipsychotics worked by blocking dopamine receptors. So it seemed obvious to most psychiatry researchers that problems with serotonin and dopamine levels must be causing depression and psychosis,” Cheyette said. “The idea that psychiatric disorders were caused by problems in brain development was a little bit radical. Now it’s hardly even a question.”
In particular, growing evidence has pointed to defective WNT signaling as a key driver of multiple psychiatric diseases. On one hand, a number of recently identified genetic contributors to schizophrenia and autism interact closely with the WNT system. In parallel, other studies have suggested that lithium salts — perhaps the oldest psychiatric drug in existence — may be successful in treating certain forms of bipolar disorder because they mimic activation of the WNT pathway in the brain.
Mutations seen in people with autism, schizophrenia, and bipolar disorder cause loss of synapses in mice
In their new paper, Cheyette and his team examined the gene DIXDC1 — a key piece of the WNT signaling pathway that is active in tissues of the brain and interacts with DISC1, a gene implicated in schizophrenia, depression, bipolar disorder, and autism spectrum disorders. Through multiple different lines of experimental evidence, they built a strong case that DIXDC1 mutations may predispose people to multiple psychiatric disorders by altering WNT signaling in the brain.
First, an analysis of genomic data from 6,000 patients with autism spectrum disorders, 1,000 patients with bipolar disorder, and 2,500 patients with schizophrenia by co-first author Pierre-Marie Martin, PhD, a postdoctoral researcher in Cheyette’s lab, revealed that disruptive mutations in the main neuronal form of DIXDC1 were present about 80 percent more often in psychiatric patients (0.9 percent had mutations) compared to healthy controls (0.5 percent had mutations).
To understand how DIXDC1 mutations put normal brain function at risk, Cheyette’s team turned to mutant mice that lacked a functioning copy of the gene. Martin conducted behavioral tests showing that, though the mutant mice were normal in many ways, they exhibited heightened anxiety, loss of motivation, and reduced interest in social interactions, all of which are reminiscent of symptoms seen in human psychiatric disorders.
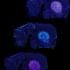
Studies of individual neurons in dishes of cultured neurons and in brain slices by co-first author Robert E. Stanley, a graduate student in the lab, revealed that neurons in the mutant mice had decreased numbers of dendritic spines, an important part of the synapses that neurons use to communicate with one another. These findings were confirmed by two-photon imaging of neurons in the brains of living mice by the lab of collaborator Yi Zuo, PhD, a neuroscientist at UC Santa Cruz, as well as electrophysiological recordings from neurons in brain slices by the lab of collaborator Vikaas Sohal, MD, PhD, an associate professor of psychiatry at UCSF.

In neurons of DIXDC1 mutant mice (center) the dendrites – neural antennae that receive input from other brain cells – have fewer of the dendritic spines (white with red arrows) — the receiving half of most synaptic inputs — compared to dendrites in wild type mice (left). Treatment of the mutant mice with lithium — the same drug used by psychiatrists for over half a century to treat patients with severe mood swings in bipolar disorder — normalizes the number of spines (right). In these images the horizontal green lines (dendrites marked with jellyfish green fluorescent protein) are each about 1/50ththe width of a human hair. [Image: Andiara Espíndola de Freitas and Robert Stanley, Cheyette Lab]
Lithium treatment restores synapse numbers, improves psychiatric symptoms in mutant mice
As expected, biochemical experiments in the Cheyette lab revealed that DIXDC1 mutations impaired WNT signaling in neurons from affected mice. Remarkably, giving animals injections of lithium salts — which mimics WNT signaling by inhibiting the molecule GSK3 — or giving animals a more specific GSK inhibitor, the researchers were able to restore normal synapse and spine numbers and also improve some of the most significant psychiatric-like behavioral abnormalities in these mice.
“That’s the key finding,” said Cheyette. “It suggests that lithium could have its well-known therapeutic effect on patients with bipolar disorder by changing the stability of spines in the brain.”
According to Cheyette, this is some of the strongest evidence to date that WNT signaling could play a key role in driving psychiatric disease, and that it works through changes in synaptic communication between neurons.
“It’s rare in psychiatry to go from human genetics to animal behavior and pharmacological rescue,” said Martin, the post-doc who co-led the research team. “Maybe each one of these lines of evidence on its own would not have been a slam dunk, but taken together, you see that all the evidence points the same way. It’s pretty compelling.”
The fact that DIXDC1 mutations were rare, even in psychiatric patients, is not a surprise to Cheyette, who says research psychiatrists now believe that — much like in cancer biology — many different risk factors combine in each individual patient to trigger neurological and psychiatric symptoms and need to be individually identified to personalize precision treatment.
“Oncologists’ growing understanding that what may clinically appear to be similar cancers can be driven by very distinct molecular pathways has led to drugs that are magic bullets for certain patients,” Cheyette said. “I think psychiatry is moving in the same direction. This paper is one more step towards a precision medicine understanding of what drives psychiatric disorders.”
Additional authors on the paper were Adam P. Ross, PhD; Andiara E. Freitas, PhD; Audrey C. Brumback, MD, PhD; Jillian Iafrati, PhD; Kristina S. Stapornwongkul; Sky Dominguez; Saul Kivimäe, PhD; Kimberly A. Mulligan, PhD; and Stephan J. Sanders, PhD, BMBS, of UCSF; Caitlin E. Moyer of UC Santa Cruz; Mehdi Pirooznia, MD, PhD, and Peter P. Zandi, PhD, of Johns Hopkins University; W. Richard McCombie, PhD, of the Cold Spring Harbor Laboratory; James B. Potash, MD, MPH, of the University of Iowa; and Shaun M. Purcell, PhD, of Mount Sinai Icahn School of Medicine.
The research was supported by the Simons Foundation Autism Research Initiative, the Brain and Behavior Research Foundation (formerly NARSAD), the National Institute of Mental Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Neurological Disease and Stroke, the Brazilian National Council for Scientific and Technological Development, and the UCSF Department of Psychiatry.
Read the research article
- Molecular Psychiatry: DIXDC1 contributes to psychiatric susceptibility by regulating dendritic spine and glutamatergic synapse density via GSK3 and Wnt/β-catenin signaling
About UCSF Psychiatry
The UCSF Department of Psychiatry and the Langley Porter Psychiatric Institute are among the nation's foremost resources in the fields of child, adolescent, adult, and geriatric mental health. Together they constitute one of the largest departments in the UCSF School of Medicine and the UCSF Weill Institute for Neurosciences, with a mission focused on research (basic, translational, clinical), teaching, patient care, and public service.
UCSF Psychiatry conducts its clinical, educational, and research efforts at a variety of locations in Northern California, including UCSF campuses at Parnassus Heights, Mission Bay, and Laurel Heights, the UCSF Medical Center at Mt. Zion, Zuckerberg San Francisco General Hospital and Trauma Center, the San Francisco VA Health Care System, and UCSF Fresno.
About the UCSF Weill Institute for Neurosciences
The UCSF Weill Institute for Neurosciences, established by the extraordinary generosity of Joan and Sanford I. "Sandy" Weill, brings together world-class researchers with top-ranked physicians to solve some of the most complex challenges in the human brain.
The UCSF Weill Institute leverages UCSF’s unrivaled bench-to-bedside excellence in the neurosciences. It unites three UCSF departments—Neurology, Psychiatry, and Neurological Surgery—that are highly esteemed for both patient care and research, as well as the Neuroscience Graduate Program, a cross-disciplinary alliance of nearly 100 UCSF faculty members from 15 basic-science departments, as well as the UCSF Institute for Neurodegenerative Diseases, a multidisciplinary research center focused on finding effective treatments for Alzheimer’s disease, frontotemporal dementia, Parkinson’s disease, and other neurodegenerative disorders.
About UCSF
UC San Francisco (UCSF) is a leading university dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. It includes top-ranked graduate schools of dentistry, medicine, nursing and pharmacy; a graduate division with nationally renowned programs in basic, biomedical, translational and population sciences; and a preeminent biomedical research enterprise. It also includes UCSF Health, which comprises two top-ranked hospitals, UCSF Medical Center and UCSF Benioff Children’s Hospital San Francisco, and other partner and affiliated hospitals and healthcare providers throughout the Bay Area.